Background: While most patients (pts) with follicular lymphoma (FL) have excellent outcomes with frontline chemoimmunotherapy (CIT), a subset of pts will experience early progression, which is associated with inferior survival. Earlier identification of high-risk FL pts could allow for intervention with novel treatments to forestall early progression. Current prognostic tools are imperfect, particularly for pts receiving bendamustine-based regimens, and novel biomarkers are needed. In Hodgkin lymphoma, interim positron emission tomography (iPET) evaluated based on Deauville score (DS) is highly prognostic and is used to guide response-adapted therapy. The prognostic value of iPET using DS has not yet been assessed in a large population of FL pts receiving frontline CIT. We hypothesized that iPET would predict progression-free survival (PFS) in this population which could support PET-guided treatment approaches.
Methods: We retrospectively identified pts with a diagnosis of FL (grade 1-3B) who initiated frontline CIT at Dana-Farber Cancer Institute from 1/2005-3/2019 and underwent an iPET after 2-4 cycles of CIT. Pts who received radiation (XRT) prior to CIT were included. Baseline, interim, and (when available) end-of-treatment (EOT) PET scans were reviewed by a nuclear medicine radiologist in a blinded fashion and assigned a DS of 1-5.
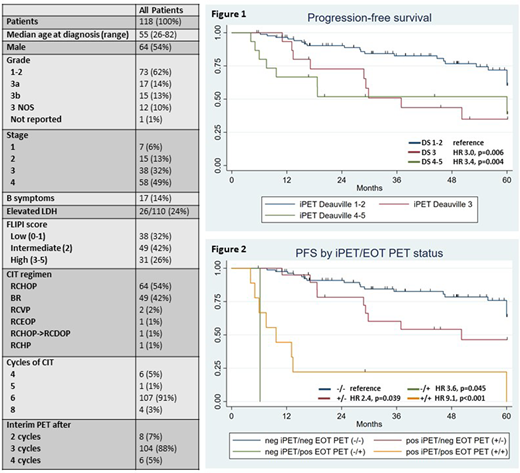
Results: 118 pts were identified. The median age was 55 (range 26-82). 73 pts (62%) had grade 1-2 FL, 17 pts (14%) grade 3A, 15 pts (13%) grade 3B, 12 pts (10%) grade 3 NOS, and 1 pt (1%) grade not reported. FLIPI score was low for 32%, intermediate for 42% and high for 26%. In total, 5 pts (4%) received XRT before CIT. The most common CIT regimens were RCHOP (54%) and BR (42%) (Table 1). 107 pts (91%) received 6 cycles of CIT and 4 pts (3%) received 8 cycles, while 7 pts (6%) discontinued CIT after 4-5 cycles due to cytopenias (4), heart failure (1), infection (1), or pt decision (1). 88% of iPETs were performed after 3 cycles. iPET DS was 1 for 18%, 2 for 57%, 3 for 13%, 4 for 9%, and 5 for 3%. EOT PET was available for review for 112 pts (95%) and demonstrated DS of 1 for 32%, 2 for 56%, 3 for 3%, 4 for 4%, and 5 for 5%. After CIT, 29 pts (25%) received a median of 9 doses (range 1-13) of rituximab maintenance (RM) and 2 pts (2%) received consolidative XRT.
With a median follow-up of 54 months (range 5-186), the 4-year (yr) PFS and overall survival (OS) for the entire cohort were 69% (95% CI 58-77%) and 94% (95% CI 87-98%), respectively. iPET was a significant predictor of PFS (p=0.0011 for 5 categories). Compared to pts with an iPET DS of 1-2, pts with a DS of 3 (HR 3.0, p=0.006) or a DS of 4-5 (HR 3.4, p=0.004) had inferior PFS (Figure 1) and were grouped together in a +iPET group (n=30) for all analyses. The 4-yr PFS for DS 1-2 and DS 3-5 pts were 77% and 46%, respectively (HR 3.2, p<0.001). iPET had similar prognostic value among pts receiving BR (HR 3.3 p=0.033) or RCHOP (HR 3.6, p=0.005) and retained significance when pts with grade 3B FL were excluded (HR 2.6, p=0.007). iPET was not predictive of OS (HR 1.6, p=0.48).
EOT PET was also a significant predictor of PFS (p<0.0001 for 5 categories). 3 pts with a DS of 3 on EOT PET had favorable outcomes and were grouped with DS 1-2 pts. A positive EOT PET (defined as DS 4-5) was observed more frequently among pts with an iPET DS of 3-5 (9/29 pts; 31%) compared to an iPET DS of 1-2 (1/83 pts; 1%) (p<0.001). To determine if iPET provides additional prognostic information beyond EOT PET, we sorted pts into 4 groups based on iPET/EOT PET status (i.e. -/-, +/-, -/+, and +/+). Compared to -/- pts, +/- pts (HR 2.4, p=0.039), -/+ pts (HR 3.6, p=0.045) and +/+ pts (HR 9.1, p=<0.001) all had inferior PFS (Figure 2). A multivariable analysis confirmed that iPET (HR 2.9, p=0.017), EOT PET (HR 7.6, P<0.001), high FLIPI (HR 2.5, p=0.011), and RM (HR 0.3, p=0.015) were significant predictors of PFS, while CIT regimen (p=0.94) and grade (p=0.21) were not.
Conclusions: Our study suggests that iPET may be a useful prognostic marker in FL. Additionally, iPET interpretation may be different in FL compared to other lymphomas. In this cohort, pts with a DS of 3 on iPET had inferior PFS with outcomes similar to those of pts with a DS of 4-5. A DS of 3-5 on iPET appears to predict earlier progression independent of EOT PET while providing response-driven prognostic information earlier in a patient's treatment course. If validated, these results suggest that iPET could be investigated as a tool for response-adapted treatment strategies in FL.
Salles:BMS/Celgene: Honoraria, Other: consultancy or advisory role; Kite, a Gilead Company: Honoraria, Other: consultancy or advisory role ; Epizyme: Honoraria, Other: consultancy or advisory role; Janssen: Honoraria, Other: consultancy or advisory role; MorphoSys: Honoraria, Other: consultancy or advisory role; Novartis: Honoraria, Other: consultancy or advisory role; Roche: Honoraria, Other: consultancy or advisory role; Abbvie: Other: consultancy or advisory role; Autolos: Other: consultancy or advisory role; Debiopharm: Consultancy, Honoraria, Other: consultancy or advisory role; Genmab: Honoraria, Other; Karyopharm: Honoraria; Takeda: Honoraria. Zelenetz:MEI Pharma: Research Funding; Celgene: Research Funding; Sandoz: Research Funding; Novartis: Consultancy; Gilead: Research Funding; Celgene: Consultancy; BeiGene: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnology: Consultancy; MorphoSys: Research Funding; Gilead: Consultancy; Genentech/Roche: Consultancy; Amgen: Consultancy; Janssen: Consultancy; Roche: Research Funding. Brown:Janssen, Teva: Speakers Bureau; Gilead, Loxo, Sun, Verastem: Research Funding; Abbvie, Acerta, AstraZeneca, Beigene, Invectys, Juno/Celgene, Kite, Morphosys, Novartis, Octapharma, Pharmacyclics, Sunesis, TG Therapeutics, Verastem: Consultancy. Crombie:AbbVie: Research Funding; Bayer: Research Funding. Davids:Ascentage Pharma: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; BeiGene: Consultancy; Celgene: Consultancy; Eli Lilly: Consultancy; AbbVie: Consultancy; Adaptive Biotechnologies: Consultancy; Genentech: Consultancy, Research Funding; Janssen: Consultancy; Bristol Myers Squibb: Research Funding; Merck: Consultancy; Research to Practice: Honoraria; Syros Pharmaceuticals: Consultancy; Zentalis: Consultancy; Sunesis: Consultancy; Gilead Sciences: Consultancy; Novartis: Consultancy, Research Funding; MEI Pharma: Consultancy, Research Funding; Surface Oncology: Research Funding; Pharmacyclics: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Verastem: Consultancy, Research Funding. Fisher:Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees. Jacobsen:Merck: Consultancy; Acerta: Consultancy; Astra-Zeneca: Consultancy; Pharmacyclics: Research Funding; F. Hoffmann-LaRoche: Research Funding; Novartis: Research Funding; Takeda: Honoraria. LaCasce:BMS: Consultancy; Research to Practice: Speakers Bureau; UptoDate: Patents & Royalties. Armand:Sigma Tau: Research Funding; Tensha: Research Funding; Pfizer: Consultancy; Affimed: Consultancy, Research Funding; IGM: Research Funding; Adaptive: Consultancy, Research Funding; Celgene: Consultancy; Merck & Co., Inc.: Consultancy, Honoraria, Research Funding; Otsuka: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Roche: Research Funding; Infinity: Consultancy; ADC Therapeutics: Consultancy; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal